Abstract
Introduction: Atrial fibrillation (AF) is not uncommon in cancer patients with grade 3-4 thrombocytopenia (platelets <50x10 9/L). The risk of bleeding appears to outweigh the risk of thrombosis in acute leukemia patients. There are no published data regarding management of anticoagulation (AC) and rates of bleeding and thrombosis in other cancer types.
Aim: To assess AC management and incidence of bleeding and thrombosis in thrombocytopenic cancer patients with AF.
Methods: Single-center retrospective cohort study. The study included adults with active cancer, grade 3-4 thrombocytopenia (platelets <50x10 9/L) and AF with CHA 2DS 2-VASc ≥1, irrespective of AC status prior index. Patients with acute leukemia were excluded. Patients were indexed when platelets <50x10 9/L. AC management was classified as either "No-AC", if AC was withheld (i.e., stopped or not started) at index, or "Continue-AC", if AC was continued. Arterial thromboembolism (ATE; ischemic stroke, transient ischemic attack or systemic embolism) and ISTH-defined major bleeding were recorded over 30 days. The 30-day cumulative incidence of composite and individual outcomes with corresponding 95% confidence intervals (CI) was calculated for each management group (death as competing risk). A Cox proportional hazards model was used to calculate hazard ratios (HR) and corresponding 95% CI for outcomes between the No-AC and Continue-AC groups, with death as a competing risk (Fine and Gray model).
Results: The eligibility criteria were met by 131 patients. At study index, AC was not given in 90 (69%) patients and continued in 41 (31%). Table 1 shows patient characteristics overall and stratified for management. The median age was 80 years )IQR 70-82) and 55 (42%) were females. Most patients were inpatients at index (70%) and had newly diagnosed cancer (70%). 64% had solid malignancy, and the remainder had hematological malignancy. The majority (92%) had AF prior to study index, while 8% had AF newly diagnosed at index. The median CHA 2DS 2-VASc score was 4 [IQR 3-5] and 18% had a prior stroke. Median platelet counts were 42 x 10 9/L at index and the median HASBLED score was 5 [3-5].
Only 44% of the No-AC group were receiving AC prior index, compared with 95% in the Continue-AC group, at shorter median duration. The type of prior AC differed between groups. Antiplatelet therapy (54%) and major bleeding prior index (13%) were more frequent in the No-AC group.
There was a median [IQR] of 4 [0-60] and 4 [1-26] days of grade 3-4 thrombocytopenia in the No-AC and Continue-AC groups, respectively. Platelet nadirs (x10 9/L) were numerically higher in the No-AC group (31 [3-50] vs. 21 [6-50]; p=0.09). A median [IQR] of 12 [6-17.25] and 10 [5-12] platelet transfusions were given to 29 (32.2%) patients in the No-AC group and 11 (26.8%) in the Continue-AC group, respectively (p>0.2). In the Continue-AC group, AC was subsequently held in 12/41 (29%) and dose-reduced in 4/41 (10%) during the 30 days post-index.
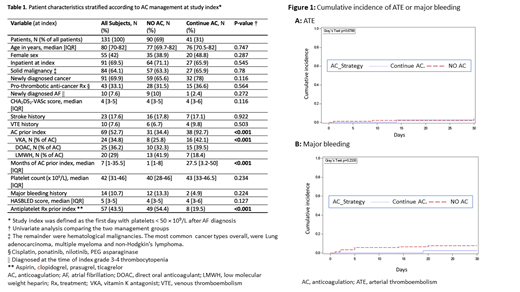
The 30-day cumulative incidence [95% CI] of the composite outcome (major bleeding or ATE) was 10% [4.88-17.27] in the No-AC group and 4.88% [0.86-14.7] in the Continue-AC group (HR 2.142 [0.47-9.609]). The 30-day cumulative incidence of ATE (Figure 1A) was 3.33% [0.88-8.66] in the No-AC group (n=3), and 4.88% [0.85-14.7] in the Continue-AC group (n=2), corresponding with a HR of 0.70 [0.12-4.10]. The 30-day cumulative incidence of major bleeding (Figure 1B) was 7.8% [3.40-14.52] in the No-AC group, and 2.44% [0.18-11.22] in the Continue-AC group (HR 3.29 [0.42-26.04]). The 30-day overall survival was 64.4% in the No-AC and 73.2% in the Continue-AC groups (HR 1.39 (95% CI 0.7-2.76).
Conclusions: In a cohort of cancer patients with grade 3-4 thrombocytopenia (<50x10 9/L) and AF (median CHA 2DS 2-VASc = 4), the majority had anticoagulation held. Baseline thrombotic and bleeding risk factors were generally balanced, but a higher rate of prior bleeding and lower rates of anticoagulation prior index in the No-AC group, suggest confounding by indication. No statistically significant difference in outcomes was detected between management groups, but 95% CI's were wide. The high bleeding and low ATE incidence in the No-AC group suggests that holding AC during time-limited periods of grade 3-4 thrombocytopenia may be a reasonable approach in many cancer patients with AF. Continuing AC should be investigated in a subset of patients with lower bleeding and higher thrombotic risk.
Falanga: Pfizer: Honoraria; Bayer: Honoraria; Sanofi: Honoraria; Leo Pharma: Honoraria. ten Cate: Bayer AG: Other; Pfizer: Other; LEO Pharma: Other; Gideon Pharmaceuticals: Other; Alveron Pharma: Other. Leader: Bayer: Honoraria; Leo Pharma: Honoraria; Novartis: Honoraria; Pfizer: Consultancy, Honoraria; Sanofi: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal